THERMAL STABILTY OF THE HYDROGEN HALIDES !!
thermal stability:
the resistance of a compound to breakdown by heating
| Hydrogen-halogen bond | Bond energy / kJ mol⁻¹ |
|---|---|
| H-F | 562 |
| H-Cl | 431 |
| H-Br | 366 |
| H-I | 299 |
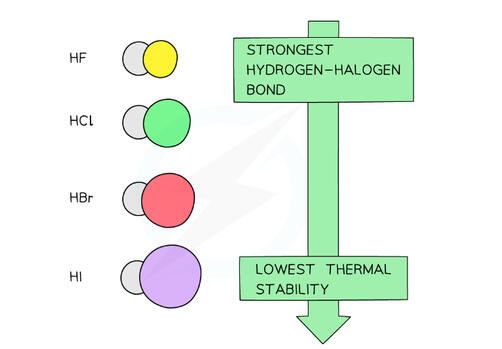
TREND
the thermal stability decreases down the group
EXPLANATION
- The decrease in the thermal stability is explained by the bond strength- The bond energies decrease down the group making it easier to break the hydrogen-halogen bond- This is because iodine is the largest atom and the overlap of its outer shell with a hydrogen atom gives a longer bond length than with the other smaller halogen atoms- The longer the bond, the weaker it is, and the less energy required to break it
| EXTRAS |
|---|
| Hydrogen fluoride and hydrogen chloride are not decomposed in temperatures up to 1500°C |
THANK YOU